News Story
Recent Clark School Graduate Featured in Lab on a Chip

Lab on a Chip journal
This June, Fischell Department of Bioengineering alumna Renee Hood (Ph.D., ’14) and a team of University of Maryland and National Institute of Standards and Technology (NIST) researchers were featured on the cover of Lab on a Chip for their paper, “A Facile Route to the Synthesis of Monodisperse Nanoscale Liposomes Using 3D Microfluidic Hydrodynamic Focusing in a Concentric Capillary Array.”
Hood is advised by Mechanical Engineering Professor and Associate Chair of Research Don DeVoe, and a member of the Maryland MEMS and Microfluidics Lab.
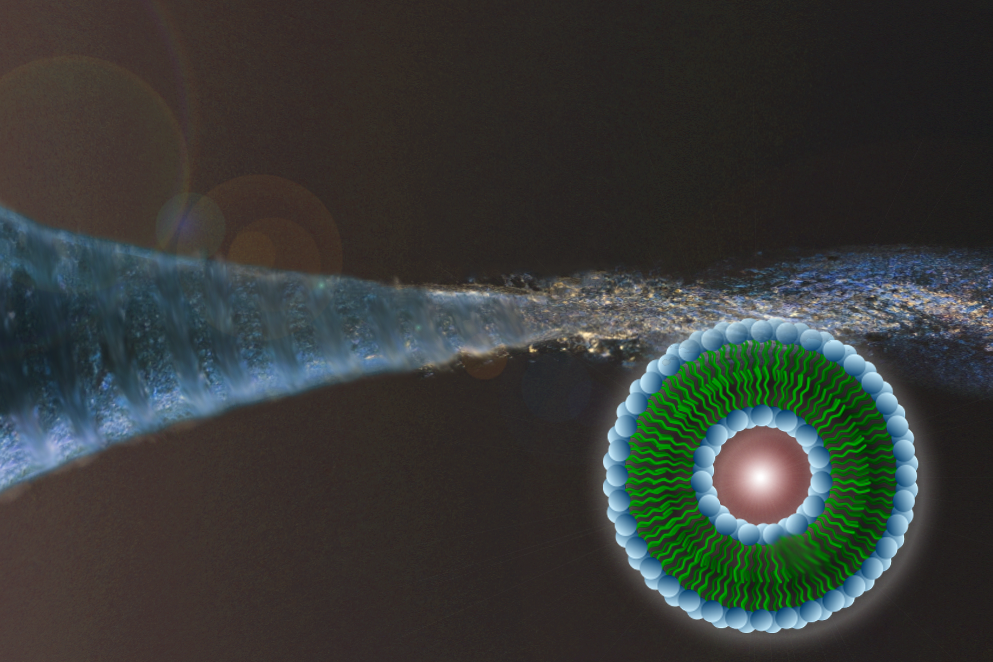
Published Tuesday, June 17, the Lab on a Chip article describes a new approach for developing sophisticated microfluidic devices to produce liquid-filled bubbles called liposomes for potential use as vehicles to deliver drugs directly to cancers and other diseased cells within the body. The group’s system is made up of bundled capillary tubes, costs less than $1 to make and requires no special fabrication technology or expertise, yet consistently yields large quantities of uniform and sturdy vesicles.
“Liposomes are biocompatible nanocapsules, which can encapsulate a range of chemically-diverse drug molecules at high concentrations, offering improved therapeutic activity and decreased toxicity compared to the drug when delivered alone,” Hood said. “The capillary-based device in this study overcomes multiple shortcomings of bulk-scale production, including size variability and tenability, as well as elevated production costs. This is significant because size and size variability are imperative factors for drug delivery and dosing. High production costs have recently caused shortages of commercially available liposomal drugs, such as Doxil®.”
The UMD and NIST researchers have therefore developed a 3D microfluidic device in which the new liposome generator consists of a 3-millimeter-diameter glass cylinder containing a bundle of seven tiny glass capillary tubes – each about the diameter of a pinhead – with one in the center and six surrounding it. A micro-sized plastic capillary (about 500 micrometers in diameter, or the length of an amoeba) is fed through the center tube and extended just beyond the end of the capillary bundle. All of the materials are commercially available at pennies per unit.
A water-based solution (known as PBS) flows through the outer six capillaries while the center channel carries the phospholipid dissolved in alcohol (in production, the PBS would carry a drug or other cargo for the vesicles). A standard glass pipette attached to the end of the microfluidic device improves mixing by concentrating the ratio of water to lipid/alcohol.
“The 3D microfluidic device we have developed through this study generates distinctly-sized liposomes at a production rate which is four orders of magnitude faster than the 2D device, and can be assembled from commercially-available materials which require no expensive laboratory infrastructure or equipment,” Hood noted.
In addition to NIST, Hood and fellow UMD researchers have collaborated with nearby groups including Children's National Medical Center and UM Baltimore School of Pharmacy, and published a number of papers in the past year.
Published June 20, 2014