News Story
Safe Storage

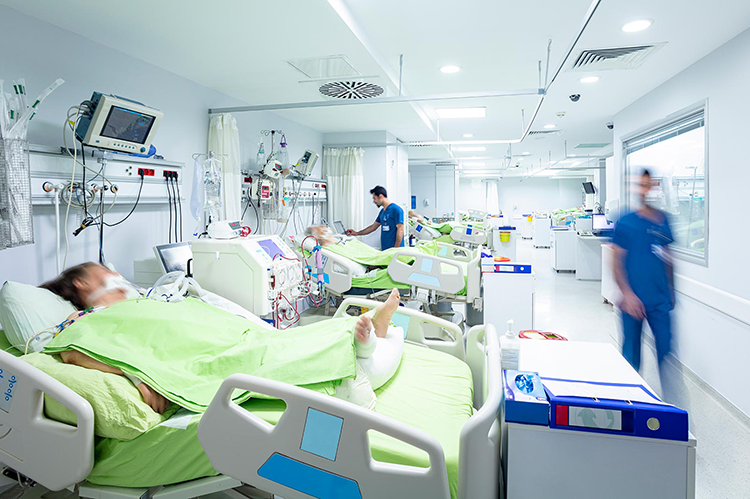
The pace was unprecedented. In December 2021, only one year after scientists had identified the virus that causes COVID-19, the first vaccines won emergency authorization. But new challenges then arose, including how to transport, distribute, and store them.
As Yunho Hwang, a research professor at the University of Maryland’s department of mechanical engineering explains, each vaccine came with its own set of specifications, including the correct temperature range for storage. Some of the vaccines had to be stored at “very low, deep-freezing temperatures,” he said.
While clinics and pharmacies were used to storing vaccines for diseases such as chickenpox or the flu, they often struggled to sort out the new requirements. It’s a challenge that continues today, as pharmaceutical companies develop hundreds of new vaccines intended to inoculate patients against the many COVID-19 variants.
To help prevent bottlenecks and costly errors, Hwang and colleagues at the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) have been working to develop a practical set of guidelines for COVID-19 vaccine refrigerated transportation and storage. The results of their work, which was undertaken on a volunteer basis out of the a desire to assist the nation’s pandemic response, are available on the ASHRAE website.
Among other types of guidance, the project aims to inform stakeholders–including, pharmacies, clinics, and ground as well as air transportation services–about the technologies they can use to meet the sometimes unusual storage needs for the new vaccines.
"We’re helping to ensure future readiness in case of public health crises."
Dr. Yunho Hwang, research professor, UMD Department of Mechanical Engineering
“The drug companies know the temperature requirements,” Hwang said. “As engineers, our job is to know what equipment is needed to meet those requirements. We try to explain what technologies are available. For example, for the lower temperatures, say -60 to -80 Celsius, a household refrigerator will not be adequate, since it can only go down to about -15 Celsuis. So special refrigeration equipment is needed.”
Without such information, clinics and other distributors may end up having to discard portions of their vaccine supply because of improper storage–and, indeed, situations like this often happened during the initial scramble to distribute the urgently-needed medicine. Hwang and his collaborators hope the guidelines they have drawn up will ensure a smoother and more effective vaccine delivery process in the future.
The work is rewarding because it has a direct benefit to society, Hwang noted. “We’re helping to ensure future readiness in case of public health crises, such as pandemics," he said.
“At the same time, I was able to collaborate with other volunteers with an interest in helping develop solutions in this area,” Hwang added.
A UMD faculty member since 1993, Hwang is co-director of the Center for Environmental Energy Engineering (CEEE), He served as chair of ASHRAE’s Refrigeration Technology Committee for Comfort, Process, and Cold Chain in 2020-2021. ASHRAE, founded in 1894, is a global society that works to advance human well-being through sustainable technology for the built environment.
Published April 13, 2022